Ocular Manifestations of Diabetes
AUTHORS:
Leonid Skorin Jr, DO, OD, MS, and Kirsten Krein, BS
ABSTRACT: Nearly half of Americans with diabetes have diabetic retinopathy, the leading cause of new cases of blindness in Americans under age 74. Despite evidence that tighter control of blood glucose and blood pressure reduces the risk of microvascular diabetes complications, as well as significant advances in the clinical management of diabetic eye disease, rates of diabetic retinopathy in the United States have increased 89% over the last decade. This article reviews the risk factors and ocular signs of diabetic retinopathy. Current concepts in therapy of diabetic retinopathy are also presented.
_____________________________________________________________________________________________________________________________________________________
Diabetes mellitus is a chronic disease with long-term macrovascular and microvascular complications. Included in these complications is diabetic retinopathy. Diabetic retinopathy is often asymptomatic, but may be evident early in the disease process. The National Eye Institute (NEI) reports that nearly half of Americans (40%-45%) with diabetes have diabetic retinopathy.1 The NEI estimates that 7.7 million Americans aged 40 and older have some degree of diabetic eye disease.1 That represents an 89% increase since 2000, and projections anticipate another jump of 75%, to 13.5 million, by 2020.1 This is thought to be due to both better detection and increasing rates of diabetes.2 Diabetes is the leading cause of new onset blindness for people aged 20 to 74 years.3
________________________________________________________________________________________________________________________________________________________________________________________________________________
RELATED CONTENT
Eye Signs of Systemic Disease: Case 6 Diabetic Maculopathy
Diabetes-Related Vision Loss a Major Issue
________________________________________________________________________________________________________________________________________________________________
The CDC have indicated that 11% of people with diabetes have some form of visual impairment with 3.8% having uncorrectable visual pathology.4 Significantly higher rates of sight-threatening diabetic retinopathy occur among African, Latino, and Native American populations.5 The duration of diabetes is the major risk factor for the development of diabetic retinopathy. Longitudinal studies found that retinopathy develops within 5 years of diagnosis of diabetes in about 25% of people with type 1 diabetes, 40% of people with type 2 diabetes who are taking insulin, and 24% of people with type 2 diabetes who are not taking insulin.6 Patients with poor glycemic control and uncontrolled hypertension are at greater risk than those with good control of these factors.7,8
How Diabetes Affects the Eye
There are many proposed mechanisms for how diabetes affects the eye; however, the exact mechanism is not fully understood. Proposed mechanisms contributing to the microvascular damage in diabetes include the direct toxic effects of hyperglycemia, sustained alterations in cell signaling pathways, and chronic microvascular inflammation with leukocyte-mediated injury.9 Whatever the mechanism or combination of mechanisms, the end result is retinal blood vessel leakage, hemorrhaging, and ischemia.
Other common nonretinal findings and symptoms of diabetic eye disease include fluctuations of refractive errors when glucose from the aqueous and vitreous diffuse into the natural lens. In cases of proliferative diabetes, neovascularization of the iris and anterior chamber angle can lead to the development of neovascular glaucoma. Extraocular muscle palsies cause diabetics to have diplopia. Premature cataracts and papillopathy of the optic nerve lead to blurred vision or even significant sight loss.10

Classifications
The following are classifications of diabetic retinopathy according to the Early Treatment Diabetic Retinopathy Study (ETDRS). The ETDRS scale is the gold standard from which a number of other grading scales have been adapted.11
Nonproliferative diabetic retinopathy (NPDR) is characterized by the presence of hemorrhages and/or microaneurysms, hard exudates, cotton wool spots (white, fluffy-appearing patches occurring in edematous areas of the retina), intraretinal microvascular abnormalities (IRMA; abnormal communication between arterioles and venules), and venous beading. This category can be further broken down into mild, moderate, and severe forms of NPDR.
Proliferative diabetic retinopathy (PDR) occurs when there is significant retinal ischemia and requires the presence of neovascularization on the retinal surface or a vitreous/preretinal hemorrhage.
Diabetic macular edema (DME) can occur independently or in addition to either NPDR or PDR. DME is diagnosed by retinal thickening and hard exudates in the macular area. DME is the leading cause of vision loss in type 2 diabetics.12 To diagnose DME, the clinician has to view the macula in 3-D. Unfortunately, the view obtained using a direct ophthalmoscope is not adequate to diagnose DME.
Risk Factors for Diabetic Retinopathy
Blood glucose control is the single most important modifiable risk factor for the development and progression of diabetic retinopathy.13 The Diabetes Control and Complications Trial (DCCT) found that in patients with type 1 diabetes mellitus for 30 years the cumulative incidence of proliferative retinopathy was 50%.14 The DCCT also reported that nearly 27% of patients with diabetes mellitus type 1 develop DME within 9 years of diabetes onset.14 For type 2 diabetes mellitus insulin-dependent patients, the 10-year incidence of proliferative retinopathy is 25.4% and for diabetes mellitus type 2 noninsulin-dependent individuals, that figure is 13.9%.14 Because almost 26 million children and adults in the United States have diabetes, a large number of patients are at risk for DME.15

Ocular Signs of Diabetic Retinopathy
Often, 1 of the first ocular signs of diabetes is microaneurysms. These occur as a result of weakened capillary walls, predisposing the small retinal vessels to leakage (Figure 1). Microaneurysms can be difficult to see, even with the high magnification of direct ophthalmoscopy. Fluorescein angiography (FA) may be necessary to truly visualize microaneurysms. FA is a procedure in which fluorescent dye is injected into the bloodstream and a series of photographs are taken with a retinal camera to evaluate the blood flow to the retina and choroid. FA is indicated when there is suspicion that a patient has neovascularization of the retina or disc and/or when DME is suspected. Intraretinal hemorrhages are another sign of diabetic retinopathy. They are referred to as dot/blot or flame-shaped hemorrhages because of their appearance. Their appearance is dictated by their location within the retina. Dot/blot hemorrhages occur in the inner layers of the retina, so the blood is spread out vertically and appears to be confined to a small area (Figures 2, 3, 4A, and 4B). Flame-shaped hemorrhages are located within the tightly packed nerve fiber layer. The blood spreads horizontally across the retina within this layer, giving the hemorrhage an elongated flame-shaped appearance (Figure 2). Cotton wool spots are areas of the retina that have become ischemic and consequently, turned a white or yellow color (Figures 2, 4A, and 4B). They represent localized nerve fiber layer swelling secondary to obstructed axoplasmic flow.16
Hard exudates are lipid residues of serous leakage from damaged capillaries and breakdown of the blood-retina barrier.17 They appear as yellow-colored deposits with sharp margins (Figures 2, 3, and 5). They can occur singularly or as a circinate ring.16 If they are present in a circular pattern, it is safe to assume there has been, or is currently, swelling of the retina within the borders of the hard exudate (Figure 3).
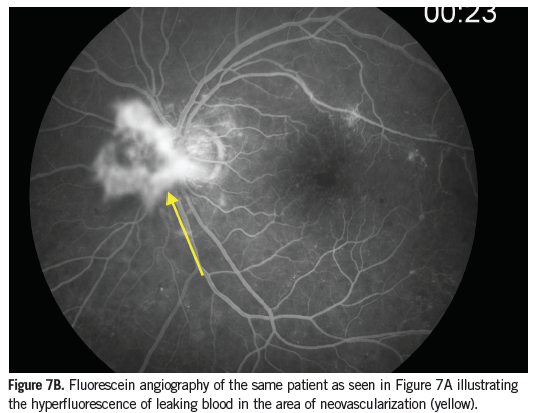
Venous beading in the retina appears as bulges in the wall of a retinal vein, giving the vessel a beaded or sausage link appearance (Figure 5). Venous beading is seen in the presence of increasing nonperfusion and is highly associated with the risk of PDR developing. IRMA are shunt vessels and appear as abnormal branching or dilation of existing blood vessels within the retina that act to supply areas of nonperfusion (Figure 3). Neovascularization can occur anywhere in the eye. If it is present along the pupillary ruff or other areas of the iris, it is referred to as neovascularization of the iris (Figure 6). If it occurs anywhere on the optic disc or if an area of neovascularization is within 1 disc diameter of the optic disc it is referred to as neovascularization of the disc (Figures 7A and 7B). Neovascularization of any size found elsewhere in the retina, greater than 1 disc diameter away from the optic disc is referred to as neovascularization elsewhere (Figure 5). Neovascularization and IRMA can be easily confused. Neovascularization has a much finer appearance when compared to IRMA and will leak when tested with FA, whereas IRMA vessels will not. In addition, IRMA lies within the retina and does not form abnormal attachments to the vitreous while neovascularization frequently does.16
These ocular signs are not necessarily in order of presentation. Although microaneurysms are almost always present if other signs of diabetes are visualized, it does not mean that hemorrhages must be present before cotton wool spots or that IRMA only occurs if hard exudates have been documented.
Diabetic retinopathy is an extremely variable disease. A patient may present with multiple retinal hemorrhages at one visit and a few months later return with virtually no signs of diabetic retinopathy. However, certain signs of diabetic retinopathy will not resolve without treatment.

Management
All patients with a diagnosis of diabetes should have a comprehensive annual eye examination with dilation. This examination should include a slit lamp evaluation for neovascularization of the iris or anterior chamber angle and for cataracts. The dilated fundus evaluation should include a magnified lens analysis to check for macular edema and binocular indirect ophthalmoscopy for evidence of retinal neovascularizaiton. Optimally, these exams are performed by eyecare practitioners. Optometrists who perform dilated eye examinations can assess the various stages of diabetic retinopathy. They would need to refer any patients with potentially sight-threatening findings to the general ophthalmologist or retinal specialist. Any evidence of macular edema or neovascular changes of the iris or retina need to be referred urgently for ophthalmologic assessment and management.
Remote diagnosis of diabetic retinopathy by the use of telemedicine (retinal cameras and photographic surveillance) in a primary care setting has the opportunity to markedly improve the annual diabetic retinopathy examination rate in a cost-effective manner.18,19 These same fundus photographs can be used as a platform for educating the patient about diabetic retinopathy and potentially improve overall diabetic compliance.
Panretinal photocoagulation (PRP) is a laser procedure that is performed when a patient has high-risk PDR and may be a viable option for patients who are
approaching high-risk PDR or who have severe NPDR. This procedure is done with the goal of preventing neovascularization from occurring or worsening, to induce regression of any existing neovascularization, and to reduce the risk of vitreous hemorrhage or tractional retinal detachment.20 It involves using a laser to make many burns in the peripheral retina (Figures 4A and 4B).
Focal or grid laser photocoagulation have been shown to reduce moderate vision loss in patients with DME by up to 50%.21 Focal laser seals leaking microaneurysms, while grid laser is used for more diffuse macular edema. Photocoagulation increases local oxygenation by reducing retinal blood flow, which results in the decrease of macular edema.22
Antivascular endothelial growth factor (anti-VEGF) drugs are quickly replacing laser as first-line treatment of DME.23 Anti-VEGF drugs target VEGF, binding to it and preventing the growth of new, weak, and permeable blood vessels. Intravitreal injections of anti-VEGF drugs are indicated when DME is present. Patients with DME should be treated before PRP is performed to the rest of the retina due to the risk of macular edema exacerbation after PRP.
Anti-VEGF injections are considered the standard of care for patients who develop DME and have best corrected visual acuity worse than 20/32.24 This treatment combined with focal or grid photocoagulation results in better visual outcomes than laser treatment alone. Delaying the photocoagulation may be beneficial in this course of treatment, as only 50% of eyes require laser treatments after 24 weeks of intravitreal anti-VEGF injections. Data shows that within 3 to 5 years of therapy, there is a 25% chance of no longer needing injections at all.25 Current anti-VEGF agents include: pegaptanib (Macugen), ranibizumab (Lucentis 0.3 mg), aflibercept (Eylea), and bevacizumab (Avastin).

Intravitreal injections of triamcinolone (Kenalog, Triescence) have been shown to decrease macular thickness and improve visual acuity in patients experiencing DME. Kenalog contains benzyl alcohol as a preservative, while Triescence does not.21 Repeat injections are needed every 3 to 4 months and have undesirable side effects in some patients. These side effects include elevated intraocular pressure, which can lead to glaucoma, as well as the accelerated formation of cataracts.27 Another option for steroid treatment of DME are the intravitreal implants dexamethasone (Ozurdex) or fluocinolone (Iluvien). The beauty of steroid implants is that they release low-dose corticosteroid for as many as 36 months with a single injection.28
Vitrectomy is indicated in diabetics who have a nonclearing vitreous hemorrhage, fibrosis, or traction that threatens the macula. Vitrectomy is an outpatient surgical procedure in which the vitreous gel is removed from the eye. Patients with a vitreous hemorrhage that has not cleared on its own after 1 to 3 months should be referred to a retina specialist for a vitrectomy.17
Conclusion
The ability to recognize these signs of diabetic retinopathy will allow for a more streamlined referral process when an ophthalmologist is required for appropriate care. If there is uncertainty, it is usually in the best interest of the patient to refer to a specialist. Patients with diabetes should have a dilated eye examination at least once a year. Those with poor glucose control or other complicating factors may be advised to have their eyes examined more often.
Leonid Skorin Jr, DO, OD, MS, is an ophthalmologist at the Mayo Clinic Health System in Albert Lea, MN.
Kirsten Krein, BS, is a fourth-year optometry student at Pacific University College of Optometry in Forest Grove, OR.
References:
1. Prevent Blindness America. 2012 fifth edition of vision problems in the US. Vision Problems US Web site. www.visionproblemsus.org. Accessed December 21, 2014.
2. National Eye Institute Statement, November 2012. Sharp rise in diabetic eye disease makes American Diabetes Month ever more important. National Eye Institute Web site.
Accessed April 2, 2015.
3. Centers for Disease Control and Prevention. 2011 national diabetes factsheet. Centers for Disease Control and Prevention Web site. Accessed January 1, 2015.
4. Centers for Disease Control and Prevention. Diabetes and eye disease. Centers for Disease Control and Prevention Web site. www.cdc.gov/visionhealth/data/national.htm. Accessed January 1, 2015.
5. Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304(6):649-656.
6. Klein R. Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care. 1995;18(2):258-268.
7. Epidemiology of severe hypoglycemia in the Diabetes Control and Complications Trial. The DCCT Research Group. Am J Med. 1991;90(4):450-459.
8. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837-853.
9. Boyer DS, Hopkins JJ, Sorof J, Ehrlich JS. Anti-vascular endothelial growth factor therapy for diabetic macular edema. Ther Adv Endocrinol Metab. 2013;4(6):151-169.
10. Holdeman NR. Diabetes mellitus. In: Onofrey BE, Skorin L, Holdeman NR, eds. Ocular Therapeutics Handbook: A Clinical Manual. 3rd ed. Philadelphia, PA: Wolters Kluwer; 2011: 398-410.
11. Grading diabetic retinopathy from stereoscopic color fundus photographs–an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(Suppl):786-806.
12. Chisiakov DA. Diabetic retinopathy: pathogenetic mechanisms and current treatments. Diabetes Metab Syndr. 2011;5(3):165-172.
13. Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556-564.
14. Progression of retinopathy with intensive versus conventional treatment in the Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. Ophthalmology. 1995;102(4):677-661.
15. American Diabetes Association. Diabetes Statistics. American Diabetes Association Web site. www.diabetes.org. Accessed December 21, 2014.
16. Hudson C. The clinical features and classification of diabetic retinopathy. Ophthalmic Physiol Opt. 1996;16(Suppl 2):S43-S48.
17. Clinical Insights. American Academy of Ophthalmology Web site. www.aao.org. Accessed December 27, 2014.
18. Wilson C, Horton M, Cavallerano J, Aiello LM. Addition of primary care-based retinal imaging technology to an existing eye care professional referral program increased the rate of surveillance and treatment of diabetic retinopathy. Diabetes Care. 2005;28(2):318-322.
19.Whited JD, Datta SK, Aiello LM, et al. A modeled economic analysis of a digital tele-ophthalmology system as used by three federal health care agencies for detecting proliferative diabetic retinopathy. Telemed J E Health. 2005;11(6): 641-651.
20. Heug LZ, Comyn O, Peto T, et al. Diabetic retinopathy: pathogenesis, clinical grading, management and future developments. Diabet Med. 2013;30(6):640-650.
21. Photocoagulation for diabetic macular edema. ETDRS report number 1. Early Treatment Diabetic Retinopathy Study Research Group. Arch Ophthalmol. 1985;103(12):1796-1806.
22. Shamsi HN, Masaud JS, Ghazi NG. Diabetic macular edema: new promising therapies. World J Diabetes. 2013;4(6):324-338.
23. Cheung N, Wong IY, Wong TY. Ocular anti-VEGF therapy for diabetic retinopathy: overview of clinical efficacy and evolving applications. Diabetes Care. 2014;37(4):900-905.
24. Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115(9):1447-1449.
25. Ho AC, Zhang J, Ehrlich JS. Ranibizumab for diabetic macular edema: long-term open-label extension of the Phase III RIDE and RISE trials. Invest Ophthalmol Vis Sci. 2014;55:1704.
26. Chan WC, Tsai SH, Wu AC, et al. Current treatments of diabetic macular edema. Int J Gerontology. 2011;5:183-188.
27. Ranchod TM, Fine SL. Primary treatment of diabetic macular edema. Clin Interv Aging. 2009;4:101-107.
28. Iluvien for Diabetic Macular Edema. Almera Sciences Web site. www.alimerasciences.com/products/iluvien-for-diabetic-macular-edema-dme.
Accessed December 29, 2014.


