Depression in the Elderly: How to Treat
ABSTRACT: Treatment of depression in the elderly can be easily initiated
and implemented in the primary care setting. If biological intervention is required, psychopharmacological therapy is relatively safe and effective even in the presence of co-occurring medical conditions. Patients usually respond to treatment, although this may take longer than it does in younger adults and it may require the combination of biological with psychological treatment, and social and spiritual interventions. Effective treatment of depression in the elderly decreases both morbidity and mortality; it also may lead to reduced demands on family members and on health care and social services.
Effective treatment of late-life depression can improve emotional, social, and physical functioning and quality of life. It has also been associated with better self-care for chronic medical conditions and reduced mortality.1
Here, in part 2 of this two-part article, the various treatment options for depression in the elderly are discussed. In part 1 (CONSULTANT, March 2012, page 225), the focus was on diagnosis.
GENERAL TREATMENT PRINCIPLES
The treatment of depression in the elderly involves biological, psychosocial, and spiritual interventions.1 The combination of biological and psychosocial intervention is moreeffective than either of these interventions alone, especially in the prevention of relapse.2 Treatment should take into account the patient’s preferences and treatment history (focusing on treatments that have been helpful in the past), and it should address coexisting medical and psychiatric conditions. Treatment availability should also be considered.
Before treatment is initiated, it is important to clarify common patient concerns about side effects, and to reassure patients that dependence is not a realistic concern with antidepressants and that these medications will not inhibit normal emotional reactions such as bereavement.3
The treatment of depression in the elderly within a primary care setting will depend on many factors, which may include:
•Treatment of co-occurring medical, psychiatric, and substance abuse
disorders.4
•Tailoring treatment interventions to the unique needs of each patient.5
•Close follow-up and frequent monitoring of both the side effects and the effectiveness of medications.6
•Consultation with mental health professionals for patients who have not responded to several pharmacological interventions or for those who prefer nonpharmacological treatment. Consultation is also recommended for patients with prominent psychotic symptoms, those with double depression (major depression superimposed on dysthymic disorder),7 and those with a complex or uncertain diagnosis.
•Psychiatric referral for patients who are candidates for electroconvulsive therapy.8
•Emergency psychiatric referral for acute suicidal ideation with intent and lethality of means.9
MANAGEMENT OF VARIOUS TYPES OF DEPRESSION
Minor depression. Patients who present with minor depression (fewer than five symptoms that persist for fewer than 2 years) may respond well to close monitoring to determine whether pharmacological treatment is necessary. Some studies have indicated that pharmacological intervention may not be effective for treating mild depressive episodes.10
Depression and co-occurring psychiatric conditions. If a patient also suffers from anxiety, treating the depression first often relieves both problems.1 More severe psychiatric disorders, such as bipolar disorder or schizophrenia, require specialized psychiatric treatment.
Depression and medical conditions. Depression can worsen many medical conditions and may even increase mortality from some disorders, such as myocardial infarction and stroke.1,11 Thus, depression should be aggressively treated in any patient with a serious medical condition.
Depression and substance abuse. Treating depression in patients who abuse alcohol or drugs is important and can sometimes help patients stop substance use. However, abstinence from substance abuse is essential for the adequate treatment of depression.1
PHARMACOLOGICAL TREATMENT
In the elderly, the average dose of antidepressants is usually one third of the typical adult dose. To prevent the emergence of adverse effects, dosages of these medications should not be increased at a rapid pace. In the elderly, the general prescribing principles described in the acronym CARE12 are of paramount importance:
•C 5 Caution, Compliance.
•A 5 Adjust dose for Age “to start low and go slow.”
•R 5 Review, Remove, Reduce.
•E 5 Educate, Educate, Educate “patients, providers, family, caregivers.”
Selective serotonin-reuptake inhibitors.The selective serotonin-reuptake inhibitors (SRIs) are most often used as first-line treatment. The SRI dosages in the elderly and side effects are summarized in Table 1.13-15
 In 2011, the US FDA informed healthcare professionals and patients that the antidepressant citalopram should no longer be used at doses greater than 40 mg/d because it can cause abnormal changes in the electrical activity of the heart.16 Studies did not show a benefit in the treatment of depression at doses higher than 40 mg/d. Changes in the electrical activity of the heart (prolongation of the QT interval of the ECG) can lead to an abnormal heart rhythm (including torsade de pointes), which can be fatal. Patients at particular risk for developing prolongation of the QT interval include those with underlying heart conditions and those who are predisposed to low levels of potassium and magnesium in the blood. These new data are highly relevant for elderly patients who are treated with citalopram.
In 2011, the US FDA informed healthcare professionals and patients that the antidepressant citalopram should no longer be used at doses greater than 40 mg/d because it can cause abnormal changes in the electrical activity of the heart.16 Studies did not show a benefit in the treatment of depression at doses higher than 40 mg/d. Changes in the electrical activity of the heart (prolongation of the QT interval of the ECG) can lead to an abnormal heart rhythm (including torsade de pointes), which can be fatal. Patients at particular risk for developing prolongation of the QT interval include those with underlying heart conditions and those who are predisposed to low levels of potassium and magnesium in the blood. These new data are highly relevant for elderly patients who are treated with citalopram.
Serotonin-norepinephrine reuptake inhibitors.If patients do not respond to the SRIs, consider the dual action serotonin-norepinephrine reuptake inhibitors (SNRIs) for treatment.The dosages and side effects of the various SNRIs are summarized in Table 2. Although head-to-head comparisons are scarce, some research suggests that SNRIs may be less well tolerated by frail elderly patients than SRIs.13,15
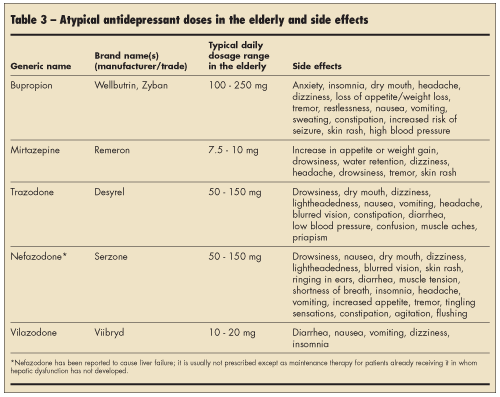
 Atypical antidepressants.Elderly patients who do not respond to SRIs or SNRIs could be treated with atypical antidepressants, which are summarized in Table 3. Because bupropion (Wellbutrin) may cause jitteriness and insomnia,17 it could be particularly useful in patients with lethargy, daytime sedation, or fatigue. It could also be used as an adjunctive treatment for tobacco cessation, since it is approved by the FDA under the trade name Zyban for the treatment of nicotine dependence. Mirtazapine is associated with sedation, increased appetite, and weight gain; thus, it may be beneficial for patients with insomnia or weight loss.18 Trazodone is usually not recommended as a primary antidepressant because of sedation and orthostatic hypotension at therapeutic doses,17,19 but in low doses it could improve insomnia associated with depression; priapism is a rare but potentially serious side effect of trazodone.20 Nefazodone has been reported to cause liver failure21; it is usually not prescribed except as maintenance therapy for patients already receiving it in whom hepatic dysfunction has not developed. Vilazodone is a newly approved antidepressant that is a combination of an SSRI and a partial agonist of serotonergic (5HT1A) receptors22; at the writing of this review, there were no specific studies about its effects on depressed elderly patients.
Atypical antidepressants.Elderly patients who do not respond to SRIs or SNRIs could be treated with atypical antidepressants, which are summarized in Table 3. Because bupropion (Wellbutrin) may cause jitteriness and insomnia,17 it could be particularly useful in patients with lethargy, daytime sedation, or fatigue. It could also be used as an adjunctive treatment for tobacco cessation, since it is approved by the FDA under the trade name Zyban for the treatment of nicotine dependence. Mirtazapine is associated with sedation, increased appetite, and weight gain; thus, it may be beneficial for patients with insomnia or weight loss.18 Trazodone is usually not recommended as a primary antidepressant because of sedation and orthostatic hypotension at therapeutic doses,17,19 but in low doses it could improve insomnia associated with depression; priapism is a rare but potentially serious side effect of trazodone.20 Nefazodone has been reported to cause liver failure21; it is usually not prescribed except as maintenance therapy for patients already receiving it in whom hepatic dysfunction has not developed. Vilazodone is a newly approved antidepressant that is a combination of an SSRI and a partial agonist of serotonergic (5HT1A) receptors22; at the writing of this review, there were no specific studies about its effects on depressed elderly patients.
 Tricyclic antidepressants. This class of antidepressants was named after its chemical structure (a three-ring molecular core). In addition to increasing levels of serotonin, tricyclic antidepressants (TCAs) affect norepinephrine, dopamine and, to varying degrees, acetylcholine and histamine. Despite their effectiveness, they are no longer considered first-line treatments because of their multiple side effects, including cardiotoxicity and lethality in cases of overdose. The most commonly prescribed TCAs are summarized in Table 4.1,23 They may be considered for patients who have previously had a good response to TCAs or who have depression that does not improve with other antidepressants. They are contraindicated in patients with a recent history (ie, within 2 weeks) of myocardial infarction, cardiac conduction defects, orthostatic hypotension, narrow-angle glaucoma, urinary retention, prostatic hypertrophy, or cognitive impairment.23
Tricyclic antidepressants. This class of antidepressants was named after its chemical structure (a three-ring molecular core). In addition to increasing levels of serotonin, tricyclic antidepressants (TCAs) affect norepinephrine, dopamine and, to varying degrees, acetylcholine and histamine. Despite their effectiveness, they are no longer considered first-line treatments because of their multiple side effects, including cardiotoxicity and lethality in cases of overdose. The most commonly prescribed TCAs are summarized in Table 4.1,23 They may be considered for patients who have previously had a good response to TCAs or who have depression that does not improve with other antidepressants. They are contraindicated in patients with a recent history (ie, within 2 weeks) of myocardial infarction, cardiac conduction defects, orthostatic hypotension, narrow-angle glaucoma, urinary retention, prostatic hypertrophy, or cognitive impairment.23
 Monoamine oxidase inhibitors. This class of antidepressants has a narrow therapeutic index and requires special dietary and medication restrictions. Their use is generally limited to patients in whom other antidepressants have failed or to patients who have previously responded well to their therapeutic effects. Consultation with psychiatrists who are experienced in prescribing these medications is advisable.
Monoamine oxidase inhibitors. This class of antidepressants has a narrow therapeutic index and requires special dietary and medication restrictions. Their use is generally limited to patients in whom other antidepressants have failed or to patients who have previously responded well to their therapeutic effects. Consultation with psychiatrists who are experienced in prescribing these medications is advisable.
The monoamine oxidase inhibitors (MAOIs) are named after the enzyme (monoamine oxidase), which breaks down many neurotransmitters, including serotonin and norepinephrine; as a result of the inhibition of the enzyme, levels of these neurotransmitters are increased. Foods that contain tyramine (eg, aged cheeses, sour cream, yogurt, cured meats, dry sausages, wine, beer, soy sauce, banana peel, liver, fava or broad bean pods, anchovies, pickled herring, caviar, yeast extracts, and sauerkraut) will interact with the MAOIs leading to a marked elevation of blood pressure, which could result in a hypertensive crisis.1 The MAOIs also interact with many other medications (eg, over-the-counter cold medicines, nasal sprays). The various MAOIs are summarized in Table 5.1,24 In addition to the adverse effects related to dietary interactions, the MAOIs have other side effects, including headache, insomnia or other sleep problems, hypotension, drowsiness, nausea, dry mouth, constipation, fluid retention, tremors, adverse sexual effects, weakness, stomach upset or pain, blurred vision, restlessness, and urinary problems.1

The MAOI selegiline, which is used as an adjunctive treatment of Parkinson’s disease, is currently available as a transdermal patch (Emsam) and has been approved by the FDA for the treatment of major depression in adults.25 Some clinicians also use it for treatment-resistant depression in the elderly. A summary of its prescription guidelines are outlined in Table 6. Unlike oral MAOIs, which exert MAO-A and MAO-B actions, transdermal selegiline is an MAO-B, which delivers antidepressant concentrations to the central nervous system without substantially impairing gastrointestinal MAO-A activity so that at the target dose of 6 mg per 24 hours, tyramine dietary restrictions are not needed.25 However, at a dose above 6 mg per 24 hours, it would lose its MAO-B selective action, thus requiring the institution of the tyramine dietary restrictions.25,26
 Side effects of transdermal selegiline include nausea, dizziness, stomach pain, drowsiness, insomnia, mild headache, dry mouth, diarrhea, increase in body movements, and vomiting.26 Localized skin reactions occur in about one third of patients and result in treatment discontinuation. Skin reactions can be minimized by proper application of the patch; persistent reactions can be treated with local corticosteroids or oral antihistamines.26 Sexual dysfunction and orthostatic hypotension, which are common side effects of oral MAOIs, are uncommon with transdermal selegiline.1,25 The recommended dosage for patients 65 years or older is 6 mg per 24 hours.
Side effects of transdermal selegiline include nausea, dizziness, stomach pain, drowsiness, insomnia, mild headache, dry mouth, diarrhea, increase in body movements, and vomiting.26 Localized skin reactions occur in about one third of patients and result in treatment discontinuation. Skin reactions can be minimized by proper application of the patch; persistent reactions can be treated with local corticosteroids or oral antihistamines.26 Sexual dysfunction and orthostatic hypotension, which are common side effects of oral MAOIs, are uncommon with transdermal selegiline.1,25 The recommended dosage for patients 65 years or older is 6 mg per 24 hours.
MAOI safety guidelines. The MAOIs are contraindicated in patients who take SRIs, SNRIs, TCAs or other antidepressants, such as bupropion, mirtazapine, buspirone, trazodone, nefazodone, and vilazodone; and certain analgesics, vasoconstrictors, sympathomimetics, and anticonvulsants.1,26 Administration of these drugs with MAOIs may cause serotonin syndrome or precipitate a hypertensive crisis. After discontinuation of any of these agents (except fluoxetine), at least four to five half-lives of the drug or active metabolite (approximately 1 to 2 weeks) should elapse before initiating therapy with MAOIs.1 Because of the long half-life of fluoxetine and its active metabolite, at least 5 weeks should elapse between discontinuation of fluoxetine and initiation of treatment with MAOIs. When switching from an MAOI to another antidepressant or other contraindicated medication, a washout period of 2 weeks is recommended.
ANTIDEPRESSANT CLASS LABELING WARNINGS
Serotonin syndrome. This syndrome is a relatively rare but potentially lethal condition caused by overstimulation of central and peripheral serotonin receptors. It typically results from interactions between medications that increase serotonergic neurotransmission. The FDA has requested a class labeling to warn about serotonin syndrome with concomitant use of SSRIs or SNRIs and triptan medications used to treat migraines, although many experts believe the risk of this adverse reaction is quite low.27
Suicidality. FDA analyses suggest that antidepressants may increase the risk of suicidal thoughts in youths and adults younger than 25 years of age.1,28 However, antidepressants have a neutral or protective effect against suicidal ideation or behavior in older adults.17 It is prudent to warn elderly patients who are receiving antidepressant therapy about the possible emergence of suicidality, to encourage all patients to quickly seek medical attention if they suffer new-onset or worsening suicidality, and to evaluate any patient with suicidality every 1 or 2 weeks. In addition, all patients who are initiating antidepressant therapy should receive follow-up within 2 to 4 weeks.
APPROACHES FOR TREATMENT-RESISTANT DEPRESSION
Augmentation and combination therapy. The addition of another antidepressant, the mood stabilizer lithium, thyroid hormone, buspirone, or a psychostimulant may be helpful for patients with refractory depression.29 Augmentation with an atypical antipsychotic medication may also be considered.1 When major depression has psychotic features, the combination of an antipsychotic with an antidepressant may be an appropriate initial strategy; however, some clinicians prefer to treat the psychosis first before adding an antidepressant.2
Combinations of antidepressants. A combination of antidepressants, usually of an SRI and a TCA, can be used in patients with refractory depression. However, patients should be monitored for toxicity from the higher plasma levels of the TCAs, which could occur because of the inhibition of cytochrome P450 induced by the SSRIs.1,17
Lithium. This mood stabilizer could be an effective augmenting antidepressant agent, and it may also decrease the risk of suicide. However, few primary care providers use lithium because of its narrow therapeutic/toxic index requiring close monitoring of lithium blood levels, especially in the setting of renal dysfunction or dehydration, which are more common in the elderly.1,30 The addition of lithium to an SRI may lead to the development of the serotonin syndrome, but this is rare and careful monitoring reduces its risk. The average dosage in the elderly is 300 to 900 mg/d in 2 to 3 divided doses; the desired serum lithium level is 0.6 to 1.2 mEq/L.
Thyroid hormones. Positive results have been reported when thyroid hormones were combined with TCAs or SSRIs in patients with treatment-resistant depression, even in the absence of hypothyroidism.1,17,31 The dosage of triiodothyronine (T3) used in this setting ranges from 5 to 12.5 mcg/d. Side effects may include palpitations, tachycardia, cardiac arrhythmias, angina pectoris, cardiac arrest, weight loss, tremors, headache, nervousness, insomnia, diarrhea, and vomiting. Decreased bone density has been reported in women who have received long-term T3 therapy. T3 is contraindicated in patients with acute myocardial infarction, thyrotoxicosis uncomplicated by hypothyroidism, and coexistence of hypothyroidism and hypoadrenalism (Addison disease), unless treatment of hypoadrenalism with adrenocortical steroids preceded the initiation of therapy for treatment-resistant depression.
Buspirone. This non-benzodiazepine anxiolytic medication has been used as augmenting adjunctive therapy for treatment-resistant depression. The main side effects include nausea, asthenia, confusion, dizziness, excitement, headache, numbness, blurred vision, feelings of anger or nervousness, and hostile behavior. Rare adverse effects are congestive heart failure, myocardial infarction, and cerebrovascular accident.1,32 The average dosage in the elderly is 5 mg 3 times a day; the dosage may be increased by 5 to 10 mg/d every 2 to 3 days, with a maximum dosage of 40 mg/d.
Psychostimulants. The efficacy of psychostimulants for the treatment of major depression remains controversial despite more than 50 years of their use in the clinical setting. Reports suggest that psychostimulants may be useful for treating medically ill patients, especially those with cancer who have depressive symptoms of severe apathy, fatigue, or psychomotor retardation.1,33 Principal adverse effects of psychostimulants include restlessness, hypertension, and tachycardia, but at lower doses adverse effects are usually not a problem. Recommended starting doses are methylphenidate 5 mg at 8 AM and noon with increases of 5 mg every 2 to 7 days. Onset of action is rapid, usually within 2 to 3 days, and in most cases, effective doses range from 10 to 40 mg per day.
Atypical antipsychotics. This class of medications can be used for the treatment of major depression with psychotic features and also as augmenting agents for treatment-resistant depression.1,14 Psychotic features may develop in about 15% of patients with major depressive disorder. Delusions and/or hallucinations often interfere with the ability to make sound judgments about the consequences of actions, which may put elderly patients at risk for harming themselves or others. The combination of an antipsychotic with an antidepressant may be an appropriate initial strategy, but some clinicians prefer to treat the psychosis first before adding antidepressants.34
The second-generation atypical antipsychotics that have been approved by the FDA as augmenting agents for treatment-resistant depression are aripiprazole (Abilify) 2 to 5 mg once daily; olanzapine plus fluoxetine (Symbyax) given at an average evening dose of olanzapine 6 mg/fluoxetine 25 mg; and extended-release quetiapine (Seroquel XR) given at an average dose of 25 to 50 mg once daily in the evening.14,35 Close monitoring is needed for potential adverse effects, such as excess weight gain, sedation, orthostasis, and extrapyramidal and anticholinergic effects.1,36 The FDA has also mandated that antipsychotic medications should carry the black box warning of increasing mortality when used to treat elderly patients with dementia because of the increased risk of cardiovascular events or upper respiratory tract infections.36,37
TREATMENT OF CO-OCCURRING PSYCHIATRIC DISORDERS
The pharmacotherapy of depression in the elderly may be complicated when there are co-occurring psychiatric disorders, such as anxiety disorders, sleep disorders, or substance abuse.
Anxiety disorders. Benzodiazepines are often prescribed for anxiety disorders; however, use of these drugs in the elderly can lead to worsening of depression, frequent falls, cognitive impairment, oversedation, increased risk of toxicity, and abuse. If benzodiazepines are needed in an acute or an emergency situation, judicious use of agents with a short half-life is preferred; monitoring for side effects, with gradual discontinuation after the anxiety symptoms have resolved, is recommended.
Sleep disorders/insomnia. Sleep disruption often accompanies depression as well as many medical conditions. This critical problem needs to be addressed, especially in elderly patients, since treatment will improve general well-being. In treating sleep disturbances in the elderly, it is important to avoid sedative hypnotics and to incorporate nonpharmacological interventions, such as sleep hygiene techniques, relaxation training and, if possible, referral for specialized individualized behavioral and/or medical treatment.
Alcohol and substance dependence and prescription medication abuse. These could precipitate depressive symptoms, which usually resolve within a few weeks of sobriety and management of psychosocial stressors. If moderate or severe depressive symptoms persist despite maintenance of abstinence and sobriety, then the pharmacological treatment of depression would need to be initiated.
PRIMARY CARE PRESCRIBING GUIDELINES
Acute treatment phase. This phase of treatment usually begins with the first dose of antidepressant medication and extends until remission of symptoms occurs; it typically lasts between 6 and 8 weeks. Close follow-up during the first 3 months of treatment iscrucial. Nearly half of patients who receive an antidepressant discontinue treatment duringthe first month, and few receive ongoing follow-up care. A minimum of three follow-up appointments duringthe first 12 weeks of antidepressant treatment may be needed to monitor progress and the emergence of side effects.4,38,39
Organized follow-up by primary care providers improves outcomes and has been shown to be cost-effective for depressed patients. Telephone follow-up may be an alternative for elderly patients who have transportation difficulties.
Failure to respond to one SSRI does not necessarily predict a lack of response to another one. No washoutperiod is necessary when switching among SSRIs or between an SSRI and a TCA. However, abruptly discontinuing a shorter-acting SSRI (such as citalopram, paroxetine, sertraline, or venlafaxine) may result in a discontinuation-interruption syndrome, which is manifested by the emergence of the initial SSRI side effects, in addition to flu-like symptoms accompanied by tinnitus, vertigo, or paresthesias.40 If a trial of a second medication is not beneficial, or if symptoms worsen, psychiatric consultation is recommended.1
Continuation treatment phase. This phase of treatment is necessary to prevent relapse and is recommended to extend to at least 6 months beyond the acute phase. During this phase, patients should be maintained at the same dose of medication used during the acute phase. Follow-up should be scheduled every 3 to 6 months. A return of symptoms indicates the need for an adjustment of the dosage, a change of medication, or a psychiatric consultation.1,38,41
Maintenance treatment phase. The goal of this phase is to avoid relapse in those patients with two or more previous episodes or with major depression lasting more than 2 years. Elderly patients in the maintenance phase need to continue pharmacotherapy for at least 2 years, and possibly indefinitely. Elderly patients with major depression who had a response to initial pharmacotherapy and psychotherapy are less likely to have recurrent depression if they receive 2 years of maintenance therapy with antidepressants.1,41,42
Medication discontinuation phase. If patients and their primary care provider agree on a trial medication discontinuation, this should be gradual, with tapering over a period of 2 to 3 months, and at least monthly follow-up, in person or by telephone. If depression recurs, prompt resumption of antidepressant medication should be initiated for at least 3 to 6 months.1,40
NONPHARMACOLOGICAL TREATMENT
Electroconvulsive therapy. Electroconvulsive therapy (ECT) remains the most effective treatment for severe and treatment-resistant depression, with an approximate recovery rate of 80%.8,43 It is well tolerated, even by the elderly, especially when there is a threat to life because of poor dietary intake or suicidal behavior, or if repeated pharmacological treatment has been ineffective. There is evidence that it is particularly effective in depression with psychotic features.43
Although there are no absolute contraindications for ECT,43,44 whenever possible hypertension and congestive heart failure should be treated before ECT is initiated. It is also advisable to postpone ECT in the first 3 months following a myocardial infarction or stroke. Elderly patients are more likely to suffer post-ECT confusion and cognitive impairment and therefore this should be carefully monitored during treatment.43,44 Memory impairment may be more frequent with bilateral electrode placement, although the response to bilateral treatment may be more rapid in its onset.43-45
Transcranial magnetic stimulation. This is a type of focal brain stimulation that has been increasingly investigated as a potential intervention for treatment-resistant depression.46Transcranial magnetic stimulation (TMS) uses magnetic fields, which involves the placement of a large electromagnetic coil against the scalp close to the forehead. The electromagnet creates painless electric currents that stimulate nerve cells in the region of the brain that is assumed to be involved in mood regulation and depression.
A newer form of TMS, rapid transcranial magnetic stimulation (rTMS), has also shown promising outcomes for the treatment of depression. The largest and most convincing database supports antidepressant efficacy for high-frequency, left dorsolateral prefrontal cortex (DLPFC) rTMS. A smaller database supports efficacy for low-frequency, right DLPFC rTMS. A more mixed database supports the use of other combinations of rTMS parameters in treating depression. However current evidence suggests that it may be less effective in the elderly, especially in the presence of even mild frontal atrophy.47
Deep brain stimulation. This surgical treatment involves the implantation of a medical device that is called a brain pacemaker, which sends electrical impulses to specific parts of the brain.48 Deep brain stimulation (DBS) in select brain regions has provided remarkable therapeutic benefits for otherwise treatment-resistant movement disorders, Parkinson’s disease, chronic pain, tremor, dystonia, and depressive disorders.49 In contrast to other surgical interventions, including lesioning and cingulotomy, DBS directly changes brain activity in a controlled manner, and its effects are reversible. This treatment modality is not recommended for treatment-resistant depression in the elderly except in rare cases in which the technique is used to treat Parkinson’s disease with co-occurring treatment-resistant depression.
Vagus nerve stimulation. Vagus nerve stimulation (VNS) is sometimes called vagal nerve stimulation; it involves a round, battery-powered generator about the size of a cardiac pacemaker that is implanted in the chest wall and attached to wires threaded along the left vagus nerve in the carotid sheath. The generator is programmed to send out electrical signals along the vagus nerve to the brain, and these signals improve depression symptoms.50 It may take several months before the effects are evident.
Possible side effects from VNS include temporary hoarseness, cough, and shortness of breath. Most of these side effects only occur during the 30 seconds that the stimulator is on. Like any operation, the implantation procedure poses some risks, including infection. As with pacemakers, the battery will need to be periodically replaced. In rare instances, damage to the device or the leads could require additional surgery prior to replacing the battery.
When VNS stimulation was approved by the FDA for the treatment of depression, it was highly publicized and marketed aggressively as a new safe intervention for treatment-resistant depression. In February 2007, the Centers for Medicare and Medicaid Services issued its preliminary decision not to cover it, citing a lack of scientific evidence of its efficacy; Blue Cross–Blue Shield had previously turned it down for similar reasons.51
Light exposure therapy.Patients with seasonal affective disorder (SAD) describe a pattern of depressive episodes in recurrent major depression or bipolar disorder.52 For many of these patients, the symptoms of depression tend to begin in the fall or winter and usually resolve in the spring. SAD can be treated with exposure to bright light (2500 lux or greater), preferably in the morning.53 Recent studies have shown that bright light treatment can reduce non-SAD depression in the elderly.54
Acupuncture.Acupuncture involves the insertion of fine needles into different parts of the body to correct the imbalance of energy in the body. At the present time there is insufficient evidence for the use of acupuncture in the elderly for treatment-resistant depression55; however, its use for the treatment of chronic pain may lead to a secondary benefit of relieving symptoms of co-occurring depression.
Complementary/alternative treatments. St John’s wort, S-adenosyl methionine (SAM-e), and omega-3 fatty acid have all been used as alternative treatments for depression.54 Patients should be advised to consult with their primary care provider before they take an herbal or other supplemental alternative agent.
St John’s wort (Hypericum perforatum). This herb is widely used in Europe for the treatment of depression.1,55 It is available over-the-counter in many pharmacies and health food stores in the United States. Although it may have some merit in treating mild to moderate depression of limited duration, it is not recommended for major depression.7
It appears to have multiple actions, including inhibition of MAO and inhibition of serotonin uptake. Many drugs interact with St John’s wort. It can increase the metabolism of some drugs, including oral contraceptives and protease inhibitors, thus reducing their effects. Because it also decreases the effects of cyclosporine, rejection of transplanted organs can occur. St John’s wort also increases sensitivity to sunlight.
S-adenosyl methionine. The production of SAM-e is impaired in persons who are depressed.1,56 It has been hypothesized that supplementation with SAM-e increases levels of serotonin, dopamine, and phosphatides, and enhances serotonin- and dopamine-receptor site binding, thus leading to amelioration of depression. SAM-e breaks down into homocysteine, elevated levels of which have been correlated with cardiac disease.1
Omega-3 fatty acid.Although it has been reported to alleviate depression, omega-3 fatty acid may exert a dose-related effect on bleeding time.1,55 Careful ongoing monitoring of bleeding time may be necessary if this supplement is used to treat depression.
PSYCHOLOGICAL INTERVENTIONS
These interventions may include psychoeducation, supportive interventions, and psychotherapy.
Psychoeducation.This intervention has been used in the elderly with good effect.57 Elderly depressed patients may view themselves as being emotionally weak or as having character defects, and primary care providers can play a vital role in helping them understand that depression results from a combination of biological vulnerability and accumulated psychosocial stressors. Providers can explain that physical symptoms can be a characteristic of depression and that effective relief of depression often makes chronic illness and physical symptoms more bearable. When patients become aware of the high prevalence of depression among the general population, they may feel less stigmatized if they are diagnosed with depression and would be more amenable to pursue treatment.
Psychoeducation also provides patients and families with information about their diagnosis, its treatment, how to recognize signs of relapse, relapse prevention, and strategies to cope with the reality of prolonged emotional or behavioral difficulties associated with aging.58 It can be a component of, or an adjunct to, other forms of psychotherapy and may be directed toward the patient or the patient’s family. The main goal is to reduce distress, confusion, and anxiety and to facilitate treatment compliance and reduce the risk of relapse.
Supportive interventions. Primary care providers could initiate interventions to alleviate the symptoms of depression by providing support and counteracting feelings of helplessness and hopelessness.1,57 This could be achieved by encouraging patients to schedule relaxing or enjoyable activities every day and by identifying exaggerated negative or self-critical thoughts. The objectives of supportive interventions are to reduce the effects of depression and to improve general physical and mental health by facilitating expansion of readily available support modalities, such as nutrition, exercise, and senior citizen centers, and by encouraging participation in leisurely activities that are designed to match the special needs of the elderly.58
Motivational interviewing and enhancing approach. Motivational interviewing and motivational enhancing techniques, which were initially developed and implemented for the treatment of substance abuse disorders and addictive behaviors, were found to be beneficial in helping patients with other psychiatric disorders, including depression.59 Primary care providers can foster this motivational process through a variety of steps, including:
•Promoting patient ownership of recommended behavioral changes (ie, getting patients to voice their own reasons for initiating change).
•Helping patients find a meaningful purpose for suggested changes.
•Formatting the specifics of behavior recommendations in a manner most consistent with patients’ personal preferences.
•Recognizing what coping mechanisms were best served by the former adverse behaviors (eg, social withdrawal, oversleeping, smoking to avoid other people) and finding alternative solutions.
•Avoiding direct confrontation and “rolling” with the patient’s resistance, thus preventing coercion or setting goals that are not readily achievable.
Psychotherapy. Psychotherapy modalities include individual, couples, family, or group therapy. Psychotherapy is usually delivered over a period of 2 to 4 months.
Supportive psychotherapy. This is a common form of therapy that may be provided over the short or long term, depending on the individual and the specific set of circumstances.60 In the elderly, this form of therapy is often useful in dealing with situational depression and loss. It is used primarily to reinforce the ability of the depressed elderly patient to cope with stressors through a number of key activities, including attentively listening and encouraging expression of thoughts and feelings; assisting in gaining a greater understanding of their situation and alternatives; improving self-esteem and resilience; and working to instill and regain a sense of hope. Generally, deeper examination of the individual’s history and probing of underlying motivation is avoided.
Interpersonal therapy. This is an exploratory intervention with a primary focus on interpersonal roles and conflicts.61 It is practical, brief, and manual-based and is applicable in the treatment of the elderly in the acute phase of depression and in relapse prevention. It focuses on disturbances in patients’ current relationships in the domains of role transition, role dispute, abnormal grief, and interpersonal conflicts. It is not intended to alter personality traits but to establish rapport and education, and it aims at improving communication, expressing feelings, and supporting renegotiated roles in relationships and problem solving. Patients who have limited options to engage in new interpersonal relationships are encouraged to tolerate problematic relationships while working in therapy to find acceptable alternatives.
Cognitive behavioral therapy. Thisis a structured therapy that is both time limited and directive, with emphasis on changing thoughts and belief systems.5,62 The goals of cognitive behavioral therapy (CBT) are to examine and modify negative thoughts, excessive self-criticism, lack of motivation, and the tendency to view problems as insurmountable. Some techniques used in CBT include challenging irrational or self-destructive thoughts, changing the way in which individuals process information, self-monitoring exercises, communication skills, problem-solving initiatives, increasing positive self-statements and experiences, and countering mistaken belief systems. Extended treatment may be necessary with long-term problems. Elderly patients should be allowed to return to therapy if their problems reoccur or if new problems arise. CBT has also shown to improve associated co-occurring medical and psychiatric conditions.
Mindfulness-based cognitive behavioral therapy. This is based on the mind-body interaction and practicing mindfulness meditation.63,64 Mindfulness meditation teaches how to focus on the present moment and act with purpose rather than letting judgments about past events or fears about what may happen in the future affect current feelings. Being focused only on the present helps patients respond positively to situations rather than react in a negative way. The use of mindfulness may better prepare elderly patients with depression to implement the changes in their negative thought patterns. The combination of CBT and mindfulness-based cognitive behavioral therapy can be an effective intervention in treating elderly depressed patients in whom CBT alone could not achieve its intended goals.
Family therapy. Depressive illness in the elderly is often complicated by enmeshed and ‘‘high expressed emotion’’ family relationships. Family therapy attempts to correct distorted communications and relationships by engaging the family in problem-centered interventions; it can lead to improved family function and enhance the recovery from the depressive symptoms.65
Music therapy. This type of therapy is usually conducted by music therapists, and it has demonstrated efficacy; it may include listening to music, playing musical instruments, dancing, and singing.66 In the elderly, music therapy could lead to improvement in depression, decreased anxiety, and remission of agitation in addition to improved coping with cognitive difficulties.
SOCIAL INTERVENTIONS
Community-based social support programs may be particularly helpful for elderly depressed patients through the implementation of strategies that ease the burden of depression.67 These strategies may include:
•Prevention of social isolation.
•Education about dividing major tasks such as shopping, house chores, etc into smaller prioritized tasks.
•Minimizing difficult roles that require a great deal of responsibility.
•Postponing major life decisions, especially financial choices, until the depressive symptoms are alleviated.
•Setting realistic expectations and not expecting to feel better in a very short period of time.
•Participating in activities that allow for relaxation and socialization, such as moderate exercise as physically tolerated. These could include aerobic activities (eg, walking, running, or bike riding) and anaerobic activities (eg, weight training and stretching).
Relaxation therapy consists of techniques of muscle relaxation and diaphragmatic breathing, which decrease physical and mental tensions, leading to improvement in depressed elderly patients, especially when their depressed mood is associated with anxiety.
SPIRITUAL INTERVENTIONS
The presence of physical illness, especially in cases of terminal illness, chronic pain, or disability, frequently complicates or exacerbates depression in the elderly. The incorporation of religion and/or spirituality into the treatment of depression could lead to more positive religious-based coping, greater strength of religious faith, and greater collaboration in problem-solving styles.68 Studies have shown that there is a high level of religion/spirituality among the elderly in the United States and a significant patient-reported desire to include such beliefs in the initial evaluation of depression in the primary care setting.69 Personal beliefs and the presence of a composite of emotional supports through religious affiliation have all been reported to be significant predictors for depression recovery beyond the effects of the initial depression severity.70
1. Khouzam HR. The diagnosis and treatment of depression in the geriatric population. Comprehensive Therapy. 2009;35(2):103-114.
2. Hollon SD. Cognitive and behavior therapy in the treatment and prevention of depression.Depress Anxiety. 2011;28(4):263-266.
3. Zisook S, Shear K. Grief and bereavement: what psychiatrists need to know. World Psychiatry.2009;8(2):67-74.
4. Birrer RB, Vemuri SP. Depression in later life: a diagnostic and therapeutic challenge. Am Fam Physician. 2004;69:2375-2382.
5. Gallo JJ, Coyne JC. The challenge of depression in late life: bridging science and service in primary care. JAMA. 2000;284:1570-1572.
6. Katon WJ, Lin E, Russo J, Unutzer J. Increased medical costs of a population-based sample of
depressed elderly patients. Arch Gen Psychiatry. 2003;60:897.
7. Devanand DP, Adorno E, Cheng J. Late onset dysthymic disorder and major depression differ from early onset dysthymic disorder and major depression in elderly outpatients. J Affect Disord. 2004;78:259-267.
8. van der Wurff FB, Stek ML, Hoogendijk WJ, Beekman AT. The efficacy and safety of ECT in
depressed older adults: a literature review. Int J Geriatr Psychiatry. 2003;18:894-904.
9. Borin L, Menon K, Raskin A, Ruskin P. Predictors of depression in geriatric medically ill inpatients. Int J Psychiatry Med. 2001;31:1-8.
10. Burke WJ, Wengel SP. Late-life mood disorders. Clin Geriatr Med. 2003;19:777-779.
11. Khouzam HR, Battista MA, Emes R, Ahles S. Psychoses in late life: evaluation and management of disorders seen in primary care. Geriatrics. 2005;60(3):26-33.
12. Williams M, Angstman K, Johnson I, Katzelnick DJ. Implementation of a care management model for depression at two primary care clinics Ambul Care Manage. 2011;34(2):163-173.
13. Coupland C, Dhiman P, Barton G, et al. A study of the safety and harms of antidepressant drugs for older people: a cohort study using a large primary care database. Health Technol Assess.2011;15(28):1-202.
14. Kelsey JE. Treatment strategies in achieving remission in major depressive disorder. Acta
Psychiatrica Scandinavica, Supplement. 2002;106(415):18-23.
15. Fava M, Rush AJ, Trivedi MH, et al. Background and rationale for the sequenced treatment alternatives to relieve depression (STAR*D) study. Psychiatric Clin North America. 2003;26(2):
457-494.
16. National Center for Biotechnology Information. U.S. National Library of Medicine. PubMed Drug
& Supplements Monograph Citalopram. Available at: http://www.nlm.nih.gov/medlineplus/druginfo/meds/a699001.html1. Accessed July 20, 2011.
17. Whooley MA, Simon GE. Managing depression in medical outpatients. N Engl J Med. 2000;343:
1942-1950.
18. Blier P, Ward HE, Tremblay P, Laberge L, Hébert C, Bergeron R. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288.
19. Fava M. Augmentation and combination strategies in treatment-resistant depression. J Clin Psychiatry. 2001;62(18):4-11.
20. Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia.
J Clin Psychiatry. 2005;66(4):469-476.
21. Khouzam HR. The antidepressant nefazodone: a review of its pharmacology, clinical efficacy,
adverse effects, dosage and administration. Psychosocial Nurs Mental Health Series. 2000;38;20-25.
22. Khan A, Cutler AJ, Kajdasz DK, et al. A randomized, double-blind, placebo-controlled, 8-week study of vilazodone, a serotonergic agent for the treatment of major depressive disorder. J Clin Psychiatry. 2011;72(4):441-447.
23. Glassman AH, Roose SP, Bigger JT Jr. The safety of tricyclic antidepressants in cardiac patients: risk/benefit reconsidered. JAMA. 1993;269:2673-2675.
24. Amsterdam JD, Hornig-Rohan M. Treatment algorithms in treatment-resistant depression.Psychiatric Clinics of North America. 1996;19(2):371-386.
25. Feiger AD, Rickels K, Rynn MA, Zimbroff DL, Robinson DS. Selegiline transdermal system for the treatment of major depressive disorder: an 8-week, double-blind, placebo-controlled, flexible-dose titration trial. J Clin Psychiatry. 2006 ;67(9):1354-1361.
26. Wecker L, James S, Copeland N, Pacheco MA. Transdermal selegiline: targeted effects on monoamine oxidases in the brain. Biol Psychiatry. 2003;
54(10):1099-1104.
27. Wenzel RG, Tepper S, Korab WE, Freitag F. Serotonin syndrome risks when combining SSRI/SNRI drugs and triptans: is the FDA’s alert warranted? Ann Pharmacother. 2008;42(11):1692-1696.
28. Coupland C, Dhiman P, Barton G, et al. A study of the safety and harms of antidepressant drugs for older people: a cohort study using a large primary care database. Health Technol Assess.2011;15(28):1-202.
29. Katon W, Unützer J, Wells K, Jones L. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. Gen Hosp Psychiatry. 2010;32(5):456-464.
30. Seemüller F, Schennach-Wolff R, Obermeier M, et al. Does early improvement in major depression protect against treatment emergent suicidal ideation? Affect Disord. 2010;124(1-2):183-186
31. Joffe RT. Hormone treatment of depression. Dialogues Clin Neurosci. 2011;13(1):127-138.
32. Khouzam HR, Emes R .The use of buspirone in primary care. Psychosocial Nursing and Mental Health Services. 2002;40(7):34-41.
33. Shelton RC, Osuntokun O, Heinloth AN, Corya SA. Therapeutic options for treatment-resistant
depression. CNS Drugs. 2010;24(2):131-161.
34. Gournellis R, Oulis P, Michalopoulou P, et al. Dimensional approach to delusions in psychotic
depression in the elderly: factor structure and clinical correlates Int J Geriatr Psychiatry.2009;24(4):
363-368.
35. Nelson J, Papakostas G. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9):980-991.
36. Gill T, Khouzam HR, Tan D. E-FALCONS: A mnemonic for monitoring the prescribing of atypical antipsychotics. Geriatrics. 2004;59(8):41-45.
37. Chahine LM, Acar D, Chemali Z. The elderly safety imperative and antipsychotic usage. Harv Rev Psychiatry. 2010;18(3):158-172
38. Gallo JJ, Bogner HR, Morales KH, et al. The effect of a primary care practice-based depression intervention on mortality in older adults: a randomized trial. Ann Intern Med. 2007;146:689-698.
39. Desai AK. Use of psychopharmacologic agents in the elderly. Clin Geriatr Med. 2003;19:697-719.
40. Yee AH, Wijdicks EF. A perfect storm in the emergency department. Neurocrit Care. 2010;
12(2):258-260.
41. Preboth M. Clinical review of recent findings on the awareness, diagnosis and treatment of depression. Am Fam Physician. 2000;15;61(10):3158, 3160, 3167-3168.
42. Unützer J, Schoenbaum M, Katon WJ, et al. Healthcare costs associated with depression in medically ill fee-for-service Medicare participants. J Am Geriatr Soc. 2009;57:506-510.
43. Tominaga K, Okazaki M, Higuchi H, et al. Symptom predictors of response to electroconvulsive therapy in older patients with treatment-resistant depression. Int J Gen Med. 2011;4:515-519.
44. Sienaert P. What we have learned about electroconvulsive therapy and its relevance for the practising psychiatrist. Can J Psychiatry. 2011;56(1):5-12.
45. O’Connor DW, Gardner B, Presnell I, et al. The effectiveness of continuation-maintenance
ECT in reducing depressed older patients’ hospital re-admissions. J Affect Disord. 2010;120(1-3):
62-66.
46. Allan CL, Herrmann LL, Ebmeier KP. Transcranial magnetic stimulation in the management of mood disorders. Neuropsychobiology. 2011;64(3):163-169.
47. George MS, Post RM. Daily left prefrontal repetitive transcranial magnetic stimulation for
acute treatment of medication-resistant depression. Am J Psychiatry. 2011;168(4):356-364.
48. Hirschfeld RM. Deep brain stimulation for treatment-resistant depression. Am J Psychiatry.2011;168(5):455-456.
49. Soulas T, Sultan S, Gurruchaga JM, Palfi S, Fénelon G. Depression and coping as predictors of change after deep brain stimulation in Parkinson’s disease. World Neurosurg. 2011;75(3-4):525-32.
50. Ondicova K, Pecenák J, Mravec B. The role of the vagus nerve in depression. Neuro. Endocrinol Lett. 2010;31(5):602-608.
51. Shuchman M. Approving the vagus-nerve stimulator for depression. N Engl J Med. 2007;356:
1604-1607.
52. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). 4th ed. Washington, DC: American Psychiatric Association; 2000.
53. Terman M. Evolving applications of light therapy. Sleep Med Rev. 2007;11(6):497-507.
54. Flory R, Ametepe J, Bowers B. A randomized, placebo-controlled trial of bright light and high-density negative air ions for treatment of seasonal affective disorder. Psychiatry Res. 2010;177(1-2):101-108.
55. Nahas R, Sheikh O. Complementary and alternative medicine for the treatment of major depressive disorder. Can Fam Physician. 2011;57(6):659-563.
56. S-Adenosyl-L-Methionine for Treatment of Depression, Osteoarthritis, and Liver Disease.Summary, Evidence Report/Technology Assessment: Number 64. AHRQ Publication No. 02-E033, August 2002. Agency for Healthcare Research and Quality, Rockville, MD.
57. Weck F, Hautzinger M, Heidenreich T, Stangier U. Psychoeducation for depression—features of interventions and therapeutic competencies. Psychother Psychosom Med Psychol. 2011;61(3-4):148-153.
58. Shimazu K, Shimodera S, Mino Y, et al. Family psychoeducation for major depression: randomised controlled trial. Br J Psychiatry. 2011;198(5):385-390.
59. Arkowitz H, Westra HA. Introduction to the special series on motivational interviewing and psychotherapy J Clin Psychol. 2009;65(11):1149-1155.
60. Funderburk JS, Sugarman DE, Labbe AK, et al. Behavioral health interventions being implemented in a VA primary care system. J Clin Psychol Med Settings. 2011;18(1):22-29.
61. Cuijpers P, Geraedts AS, van Oppen P, et al. Interpersonal psychotherapy for depression: a meta-analysis. Am J Psychiatry. 2011;168(6):581-592.
62. Chong MS, Chan MP, Kang J, et al. a new model of delirium care in the acute geriatric setting: geriatric monitoring unit. BMC Geriatr. 2011;13;11(1):41.
63. Piet J, Hougaard E. The effect of mindfulness-based cognitive therapy for prevention of relapse in recurrent major depressive disorder: A systematic review and meta-analysis. Clin Psychol Rev. 2011;31(6):1032-1040.
64. Khouzam HR. Chronic fatigue syndrome: an update on treatment in primary care. Consultant.2011;51(3):145-150.
65. Andreescu C, Reynolds CF 3rd. Late-life depression: evidence-based treatment and promising new directions for research and clinical practice. Psychiatr Clin North Am. 2011;34(2):335-55, vii-iii.
66. Maratos A, Crawford MJ, Procter S. Music therapy for depression: it seems to work, but how? Br J Psychiatry. 2011;199:92-93.
67. Ayalon L, Shiovitz-Ezra S. The relationship between loneliness and passive death wishes in the second half of life. Int Psychogeriatr. 2011;22:1-9.
68. Nasser EH, Overholser JC. Recovery from major depression: the role of support from family, friends, and spiritual beliefs. Acta Psychiatr Scand. 2005;111:125-132.
69. Stanley MA, Bush AL, Camp ME, et al. Older adults’ preferences for religion/spirituality in
treatment for anxiety and depression. Aging Ment Health. 2011;15(3):334-343.
70. Hofmann SG, Grossman P, Hinton DE. Loving-kindness and compassion meditation: Potential
for psychological interventions. Clin Psychol Rev. 2011;31(7):1126-1132.
Acknowledgments: The author thanks Dr Mohammed Shaalan for his inspiration; Dr Avak Howsepian for his constructive criticisms; the VA Central California Health Care System Hospital Director, Mr Alan Perry, FACHE, for his administrative and leadership support; the chief of staff, Dr Wessel Meyer, for his clinical support; Drs Robert Hierholzer, Nestor Manzano, Scott Ahles, and Craig C. Campbell, for their academic guidance; and Dr Matthew Battista, PhD, and Mr Leonard Williams, PA, for their encouragement.


